KABUL (Pajhwok): Laboratory test result of the Genlide Azithromycin capsules proved negative and the drug was no more useful, according to the Afghanistan Food and . . .
You need to subscribe to view the full article. Please login or register a new account.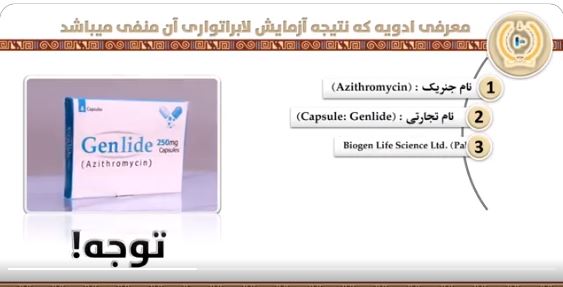






GET IN TOUCH
NEWSLETTER
SUGGEST A STORY
PAJHWOK MOBILE APP